Abstract
Background: Peripheral T-cell lymphomas (PTCLs) represent a heterogeneous disease group accounting for 10% of non-Hodgkin lymphoma. Approximately 25-30% of patients present with stage I or II disease. The natural history and outcomes of patients with limited stage PTCL is poorly defined. Considering the paucity of literature, the purpose of this study was to assess the outcome of patients with limited stage PTCL, to investigate the potential prognostic factors and build a prognostic model specific for limited stage PTCLs.
Methods: The International T Cell Project database (TCP 1) is a prospective registry that collected clinical data and biological information in >1,500 cases with PTCLs at 75 institutions worldwide between 2006-2018. The TCP was searched for all patients aged >18 years diagnosed with PTCL NOS, AITL or ALK+ or ALK- ALCL with stage I or II disease. Survival was updated until database lock on 30 March 2019. The primary end point was overall survival (OS) at 5 years measured from the date of diagnosis until death from any cause or the date of last known contact for living patients. Cox models were used to investigate the association between survival outcomes and covariates with hazard ratios used as a summary measure. Covariates identified to have an independent prognostic effect on OS were used to create a novel prognostic model (Limited Stage Peripheral T Cell Lymphoma Prognostic Model)(SALENTO Model). An external validation cohort of patients with newly diagnosed PTCLs registered in the Australian Lymphoma and Related Diseases Registry (LaRDR) and the Brazilian T-Cell Lymphoma Registry (BrTCLR) was used to validate the predictive model. The performance of the Salento model was compared to published prognostic indices: Prognostic Index for T-cell lymphoma (PIT), Prognostic Index for T-cell lymphoma and platelets (IPTCLP) using Akaine information criterion (AIC) and concordance index (c-Harrell). High-risk disease was defined as Salento≥2; PIT≥2 or IPTCLP≥2 as previously reported. Statistical analyses were performed using Stata (version 14.2) and SPSS (version 20.0).
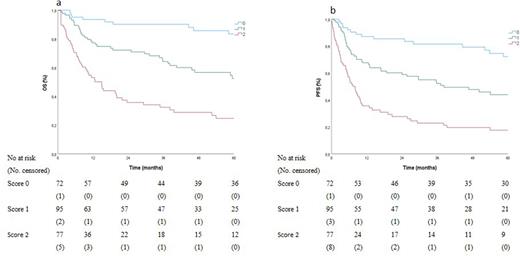
Results: 244 patients registered in the TCP fulfilled the inclusion criteria: PTCL-NOS (n=129), AITL (n=28), ALCL ALK+ (n=33) and ALCL ALK- (n=54). The median age was 57 years (range 18-88). 199 pts (81%) received anthracycline-based regimens, 27 pts (11%) radiotherapy alone (RT), 8 pts (3%) chemotherapy+RT and 12 pts (5%) best supportive care. After a median follow-up of 52 months, the 5-year OS and progression-free survival (PFS) rates were 52% and 43%, respectively. Age >60y, elevated LDH and low serum albumin were independent prognostic factors. The resulting score identified three groups with low- (LR, 29% pts, score 0), intermediate- (IR, 39% pts, score 1), and high-risk (HR, 33% pts, score 2-3) with 5-yr OS of 83% [95% CI 31-100], 53% [95% CI 27-71], and 25% [95% CI 23-84], respectively (P<0·001)(Figure - left panel) and 5-yr PFS of 72% [95% CI 35-100], 44% [95% CI 9-68], and 17% [95% CI 9-35], respectively (P<0·001)(Figure - right panel).
110 patients registered in the LaRDR/BrTCLR fulfilled the inclusion criteria and represented an external validation cohort. The median follow-up of the validation cohort was 22 months (range 20-27 months), and 52 events (53% of patients) were recorded. The model was therefore tested in terms of 2-yr OS: 27 patients (23%) LR, 53 patients (49%) IR; and 30 patients (31%) HR. The distribution of the different risk groups was almost superimposable between the training and validation cohorts. The 2-year OS of the three groups was 95% [95% CI 82-ne], 81% [95% CI 74-88] and 52% [95% CI 23-812] for the LR, IR and HR, respectively (P<0,001, HR=3.55, 95% CI 1.7-7.2) in the validation cohort.
In comparison to the PIT and IPTCLP, the Salento model demonstrated the greatest discriminant power with a lower AIC (512) and higher Harrell C-statistic (0.701) than the other prognostic indices (Harrell C- statistic 0.641 for PIT and 0.528 for IPTCLP).
Conclusions: The Salento Model (Limited Stage Peripheral T Cell Lymphoma Prognostic Model) is an objective, simple and robust prognostic tool. It demonstrated greater discriminatory power than established prognostic indices and an analogous distribution and outcomes in the validation cohort. The model identifies a high-risk group with poor outcomes, comparable to advanced stage disease, that should be considered for innovative first-line approaches.
Disclosures
Vose:Janssen: Honoraria; Lilly: Honoraria; AbbVie: Honoraria; Johnson & Johnson: Consultancy; Daiichi Sankyo: Consultancy; AstraZeneca: Honoraria; Kite, a Gilead Company: Research Funding; Pharmacyclics: Consultancy; MorphoSys: Consultancy. Advani:ADC Therapeutics, Cyteir, Daiichi Sankyo, Gilead, Merck, Regeneron, Roche, Seattle Genetics: Research Funding; ADC Therapeutics, BMS, Daiichi Sankyo, Epizyme, Gilead, Incyte, Merck, Roche, Sanofi: Consultancy. Horwitz:Affimed,: Consultancy; Takeda: Consultancy; SecuraBio: Honoraria; Shoreline Biosciences, Inc.: Membership on an entity's Board of Directors or advisory committees; C4: Research Funding; Verastem/SecuraBio: Research Funding; Kyowa Hakko Kirin: Research Funding; Daiichi Sankyo: Research Funding; Crispr Therapeutics: Research Funding; ADC Therapeutics: Research Funding; Celgene: Research Funding; Yingli Pharma Limited and Tubulis: Honoraria; ONO Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Kyowa Hakko Kirin: Consultancy; Affimed: Research Funding; Seattle Genetics,: Research Funding; Millennium /Takeda: Research Funding; Daiichi Sankyo: Membership on an entity's Board of Directors or advisory committees; Cimieo Therapeutics: Honoraria. Foss:Daiichi: Consultancy; Kyowa: Consultancy; Conjupro: Consultancy; Astex: Consultancy; Seagen: Consultancy, Speakers Bureau. Radford:Astrazenca: Current equity holder in private company, Current holder of stock options in a privately-held company; ADC Therapeutics: Consultancy, Current equity holder in private company, Current holder of stock options in a privately-held company, Honoraria, Speakers Bureau; Takeda: Consultancy, Research Funding, Speakers Bureau; BMS: Consultancy, Honoraria; Kite Pharma: Consultancy; The University of Manchester and Christie Hospital NHS Foundation Trust: Current Employment. Chiattone:Takeda Oncology Brazil: Research Funding. Kim:Boryong: Research Funding; Roche: Research Funding; Kyowa-kirin: Research Funding; Merck: Research Funding; Sanofi: Research Funding; Beigene: Research Funding. Trotman:Roche: Research Funding; Beigene: Research Funding; Takeda: Research Funding; Cellectar: Research Funding; BMS: Research Funding; PCYC: Research Funding; Janssen: Other: clinical trials. Luminari:Genmab: Consultancy; Regeneron: Consultancy; BMS/Celgene: Consultancy; Gilead/Kite: Consultancy; Janssen: Consultancy; Roche: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal